Absolute and Relative Pressure
Pressure is a fundamental concept in physics, referring to the force exerted by a fluid or gas per unit area. In the case of a gas container, pressure is generated by the movement of gas molecules colliding with the walls of the container. Although the molecules are small, there are many molecules in a modest-sized container, which means that the collision with the walls is constant and uniform in all directions.
When we compress a gas, the pressure and the temperature increases due to the friction between the molecules that are forced closer together. Compression reduces the volume and increases the pressure, resulting in a stronger interaction between the molecules and therefore a higher temperature. If a pressure gauge were placed in the container before and after compression, the pressure would be observed to rise.
Absolute pressure refers to the measurement of the pressure inside a vessel relative to an absolute vacuum. If a manometer is in a vacuum, it does not interact with any gas molecules and its reading will be zero. If the vessel is charged with five bar of pressure, the manometer will measure five bar, since there is nothing affecting it in a vacuum.
Relative pressure, on the other hand, is measured with a manometer that is already subjected to an opposite pressure, such as atmospheric pressure, which is the weight of the column of air above us. If the gauge measures zero bar and the vessel is loaded with five bar, there will be an opposite pressure of one bar, resulting in a reading of four bar on the gauge.
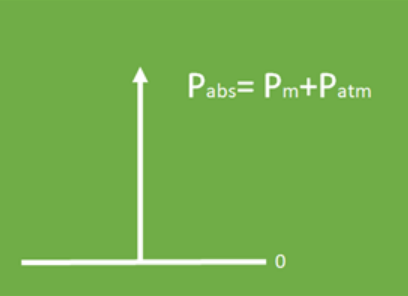
It is important to note that absolute pressure is the sum of gauge pressure and atmospheric pressure. The gauge pressure is the measurement obtained with a manometer and the atmospheric pressure is the force that the air exerts on the surface of the Earth. The difference between absolute pressure and gauge pressure is the atmospheric pressure.
In short, right now, as you read this text, your body is subjected to a pressure of one bar, one atmosphere. However, in a completely sealed refrigeration circuit, the atmospheric pressure has no influence, “it has its own pressure”. When measuring this refrigerant circuit pressure, we measure it with a manometer, which is pressurised to one bar, one atmosphere, and this interferes with its measurement. The pressure of the refrigeration circuit, without the influence of the atmosphere, is an Absolute Pressure and the pressure measured by the manometer, influenced by the atmospheric pressure, is a Relative Pressure.
More articles
Interested in other (technical) knowledge articles? Keep yourself up to date and read them all

