Sensible and Latent Heat
In this article we will explore the concepts of heat and temperature, and within the concept of heat, we will examine two types: latent heat and sensible heat. It is essential to understand the difference between them in order to understand how Heat Pump equipment works.
Heat is based on the internal energy of the molecules of a body, which increases when the molecules move with more force. On the other hand, we must be clear that cold is not a concept in itself, but the absence of heat in a body. Albert Einstein stated that both cold and darkness are invented concepts that do not exist.
Temperature is a measure of the internal energy of a body. When the internal energy increases, the temperature increases. When two bodies with different temperatures are brought together, the heat transfer will always be from the hotter body to the colder one. Never the other way around.
Specific heat is the amount of heat required to raise the temperature of a substance by one degree. Air has a low specific heat, which means that it requires little energy to raise its temperature. Water, on the other hand, has a high specific heat, which means that it takes a lot of energy to raise its temperature.
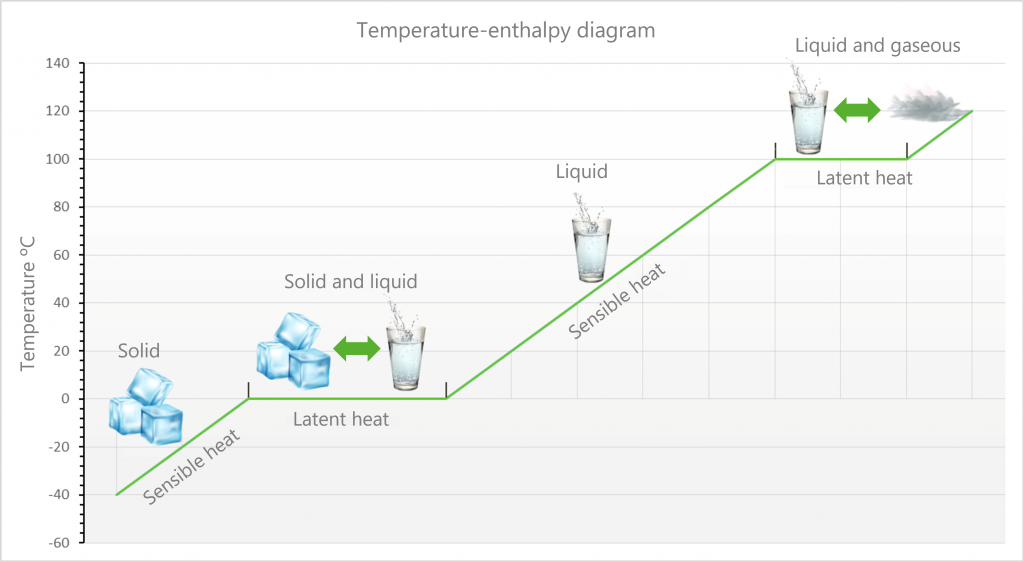
Sensible heat changes the temperature of a body, whereas latent heat involves a change of state without a change in temperature.
We usually assume when heat is added to or subtracted from a substance, its temperature also changes, it warms up or cools down, but this is not always the case. If the substance undergoes temperature changes when heat is added or subtracted, it is sensible heat. A soft drink in a refrigerator loses heat, lowing its temperature, it is a sensible heat.
If it does not undergo temperature changes, it is latent heat, only phase change. When an ice cube melts in a soft drink, it picks up heat from the liquid, but the ice remains at the same temperature 0°C, this heat is latent, the water changes from ice to liquid, the phase changes.
Understanding the difference between sensible heat and latent heat is fundamental to understanding the operation of Heat Pump equipment. In the condenser, there is a change of temperature, which involves sensible heat, while in the evaporator, there is a change of state, which involves latent heat. Herein lies the need to understand the difference between the two in order to understand how Heat Pump equipment works.
More articles
Interested in other (technical) knowledge articles? Keep yourself up to date and read them all

